Chapter 1 The Foundations of Biochemistry
1 Biochemistry is a science dealing with the chemistry of life
Life is characterized by
- High degree of chemical complexity and microscopic organization
- Extraction, transformation, and systematic use of energy to create and maintain structures and to do work
- The interactions of individual components being dynamic and coordinated
- Ability to sense and respond to changes in surrounding
- A capacity for fairly precise self-replication while allowing enough change for evolution
2 Cells are the structural functional units of all living organisms
- Living organisms are made of cells
- Simple living organisms are single-celled
- Larger organisms consists of many cells with different functions
- All cells of the simplest organisms and the most complex organisms share certain fundamental properties at the biochemical level
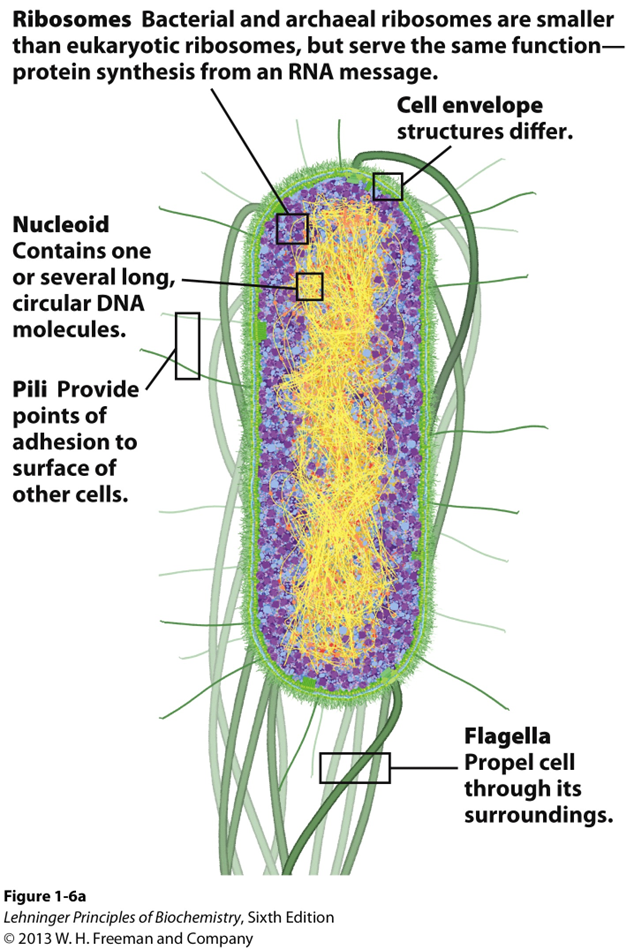
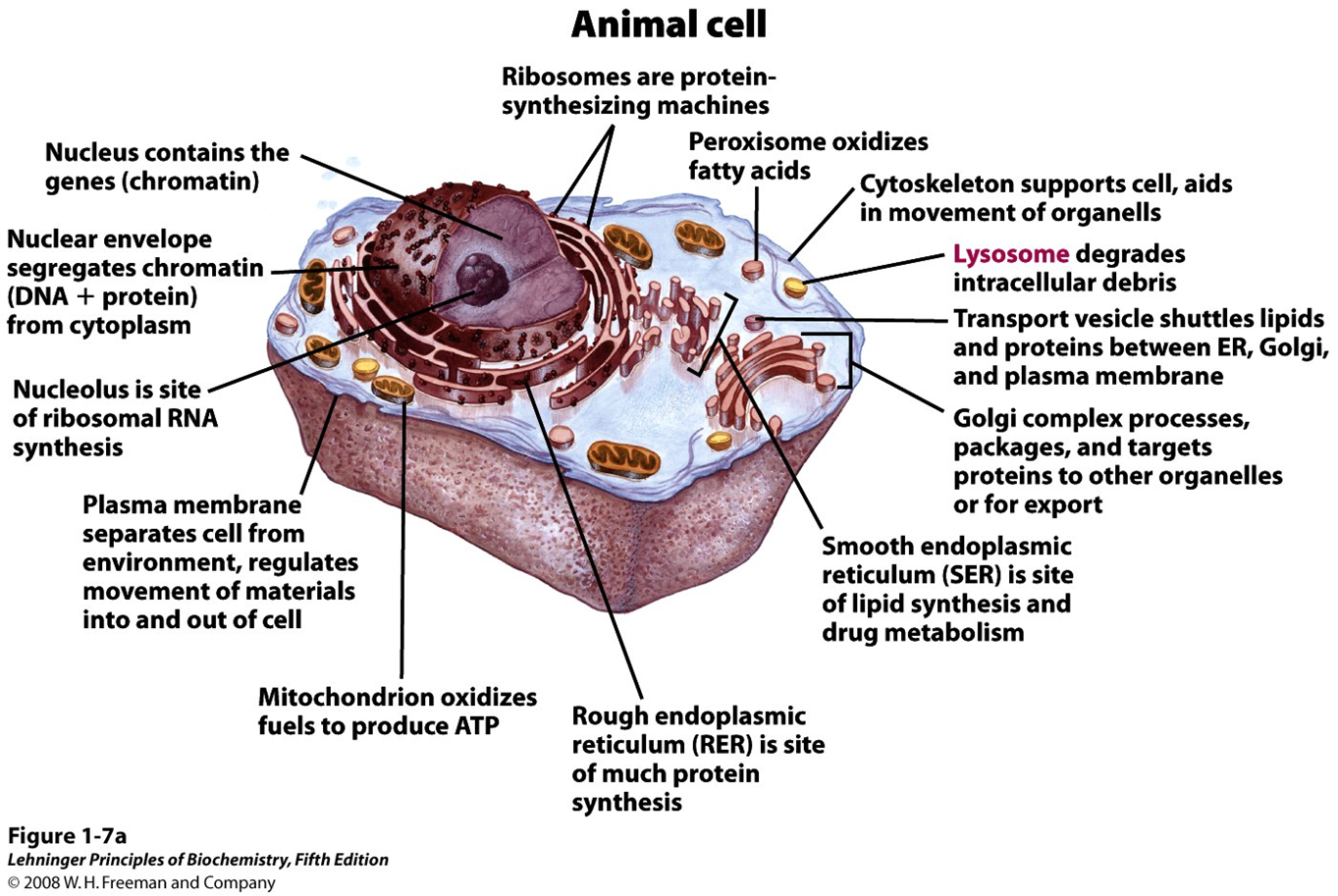
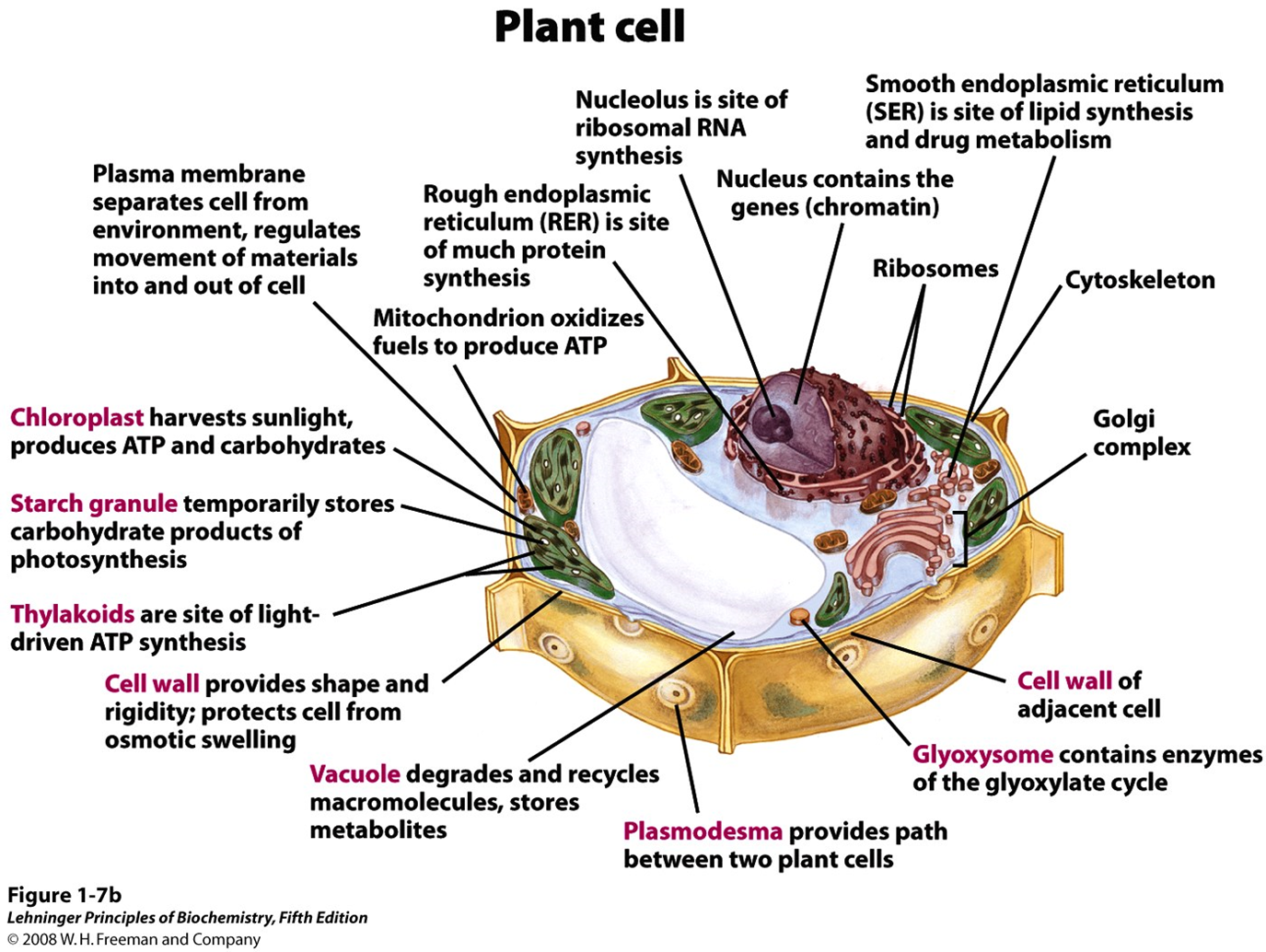
Intro to Cytoplasm and cytoskeleton
- Cytoplasm is highly viscous solution where many reactions take place
- Cytoskeleton consists of microtubules, actin filaments, and intermediate filaments
- cellular shape and division
- intracellular organization
- intracellular transport paths
- cellular mobility
3 The molecular logic of life
Biochemistry aims to explain the chemistry that is behind the:
- initiation and acceleration of reactions
- organization and specificity of metabolism and signaling
- storage and transfer of information and energy
Some common functional groups of biomolecules
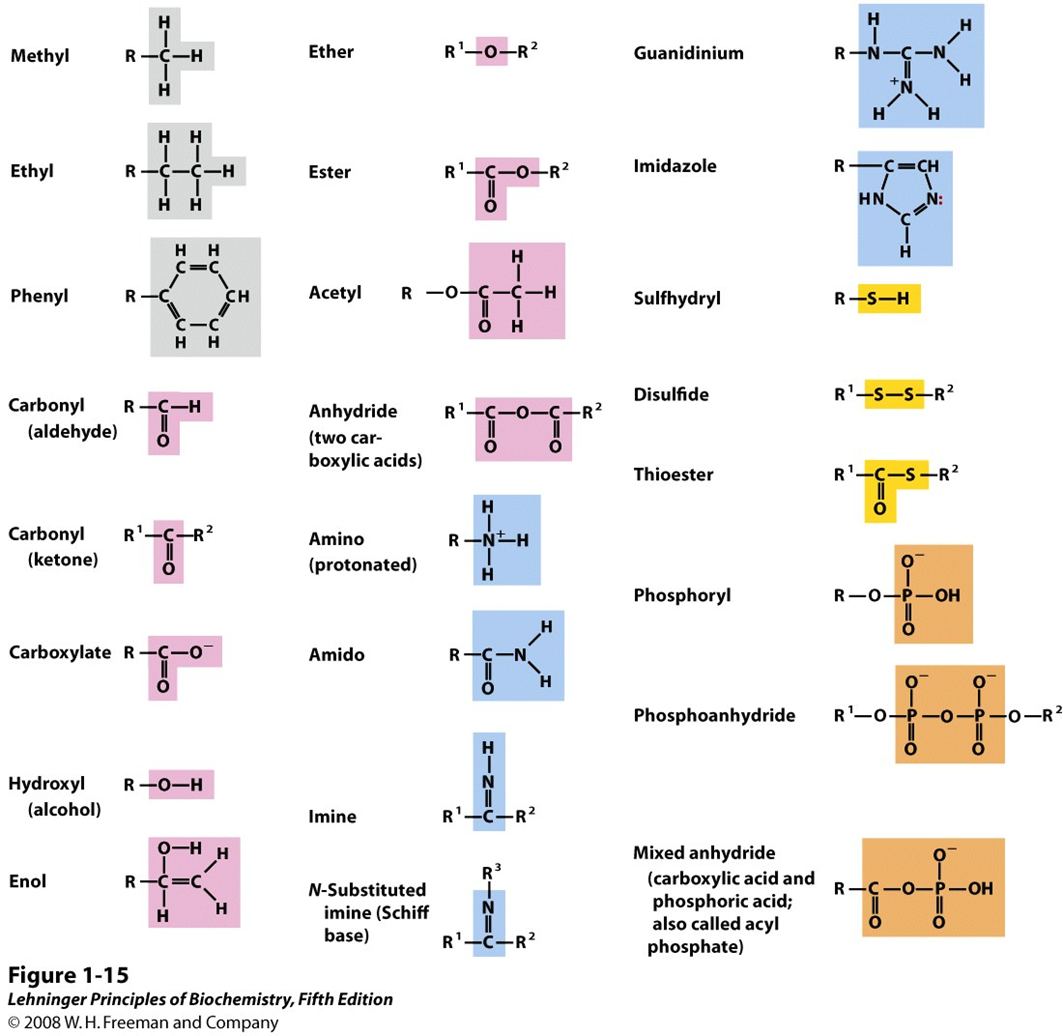
Important: Carbonyl(aldehyde), Carbonyl(ketone), Carboxylate, Hydroxyl, Acetyl, Amido, Disulfide, Thioester, Phosphoryl
Structure of biological molecules
- Configuration (构型)
- the fixed spatial arrangement of atoms in a molecule
- Conformation (构象)
- the spatial arrangement of substituent groups that are free to assume different positions in space without breaking any bonds, because of the freedom of rotation about single bonds
Stereoisomers have different biological properties. Interactions between biomolecules are stereospecific.
Structural hierarchy in the molecular organization of cells
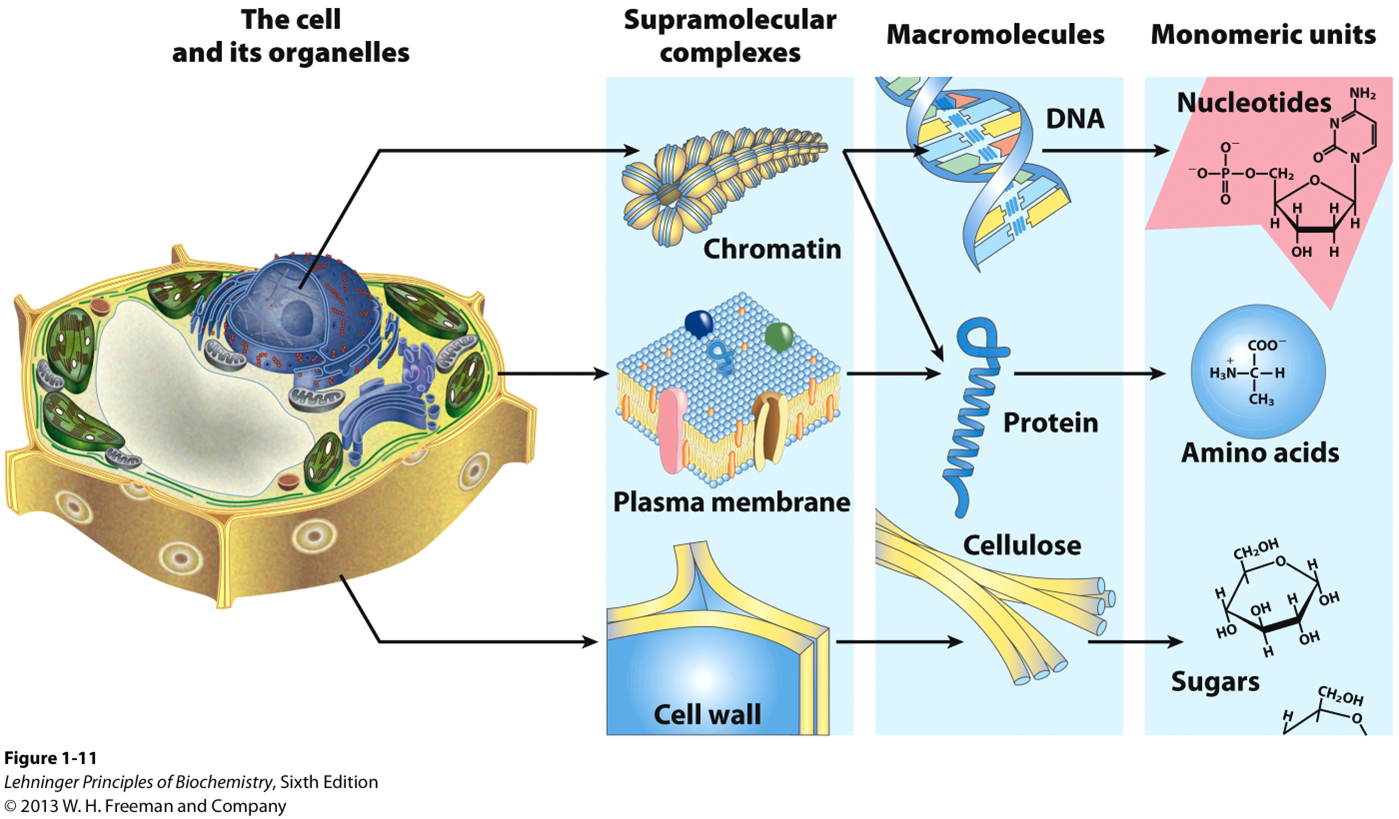
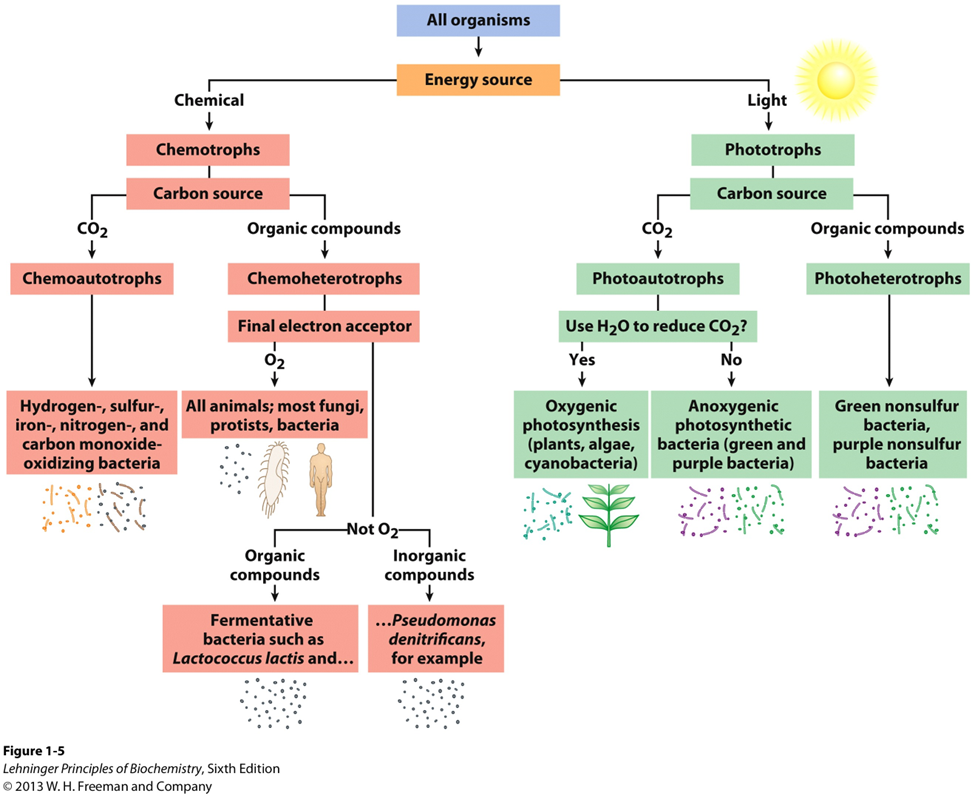
4 All organisms require energy and carbon for life
Organisms can be classified according to their source of energy (sunlight or oxidizable chemical compounds) and their source of carbon for the synthesis of cellular material.
ATP(Adenosine triphosphate) is the chemical currency of energy.
- Synthesis of complex molecules and many other metabolic reactions requires energy (endergonic)
- Breakdown of some metabolites releases significant amount of energy (exergonic)
How to speed up reactions
- Higher temperatures
- Higher concentration of reactants
- Coupling the reaction to an exergonic one
- Lower activation barrier by catalysis
The last two are used by living organisms
Enzymes lower the activation free energy and offer
- Acceleration under mild conditions
- High specificity
- Possibility for regulation
Series of related reactions forms a pathway
- Metabolic Pathway:
- produces energy or valuable materials
- Signal Transduction Pathway:
- transmits information
Pathways are controlled in order to regulate levels of metabolites.
5 Genetic and evolutionary foundations
The central “dogma”
Pathway for the flow of genetic information: DNA → RNA → Protein
- DNA: stores information
- RNA: transmits information
- Protein: function manifests information
Natural selection favors some mutations
RNA can act both as the information carrier and biocatalyst
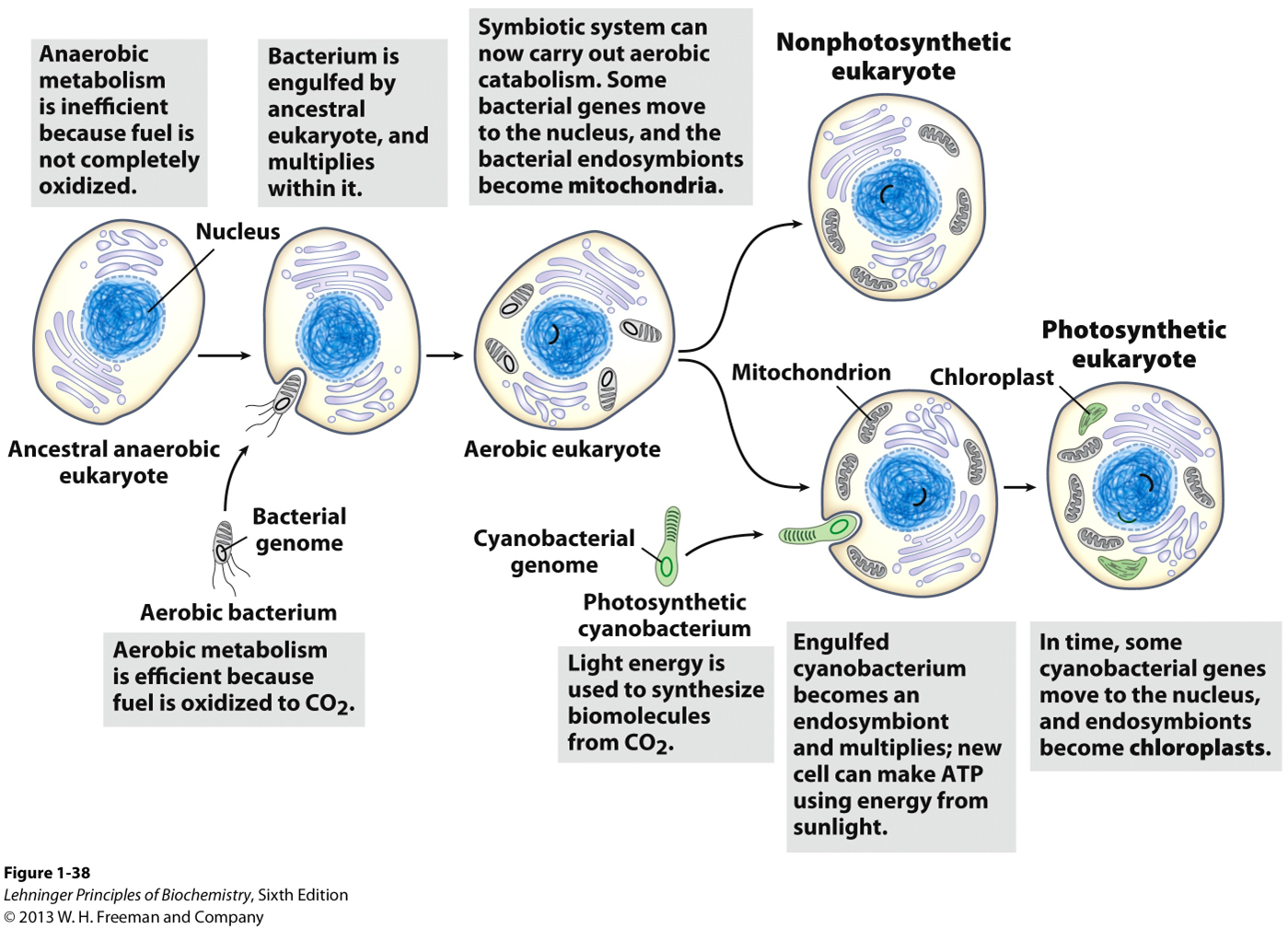
Evolution of eukaryotes through endosymbiosis
Summary
- Cells are the structural and functional units of all living organisms.
- Cells contain a universal set of small molecules.
- Macromolecules are the major constituents of cells.
- Structure of biological molecules is important for their function.
- Interaction between biological molecules are stereospecific.
- Energy coupling links reactions in biology.
- The linear sequence in DNA encodes proteins with three-dimensional structures.
Chapter 2 Water
1 Water is the medium for life
- Life evolved in water
- Organisms typically contain 70-90% water
- Chemical reactions occur in aqueous environment
- Water is both a solvent and a reactant
- Water is a critical determinant of the structure and function of proteins, nucleic acids, and cell membranes.
There is electrostatic attraction between the O atom of one H2O molecule and the H atom of another H2O molecule — hydrogen bond. Hydrogen bond is weaker than Covalent bond.
2 Hydrogen bond
- Weak electrostatic interaction between one electronegative atom (the H acceptor, frequently N and O) and a hydrogen atom covalently bonded to a second electronegative atom (the H donor)
- The hydrogen acceptor is usually oxygen or nitrogen; the hydrogen donor is another electronegative atom.
- Typically 4-6 kJ/mol for bonds with neutral atoms, and 6-10 kJ/mol for bonds with one charged atom
- Hydrogen bonds are strongest when the bonded molecules are oriented to maximize electrostatic interaction. Ideally, the three atoms involved lie in a straight line.
Importance of hydrogen bonds
- Source of unique properties of water
- Structure and function of proteins
- Structure and function of DNA
- Structure and function of polysaccharides
- Binding of substrates to enzymes
- Binding of hormones to receptors
- Matching of mRNA and tRNA
H2O can serve as both an H donor and an H acceptor. H2O is a polar molecule, capable of forming hydrogen bands with itself or with other molecules. And Up to four hydrogen bonds per H2O molecule gives H2O
- anomalously high boiling point
- anomalously high melting point
- unusually large surface tension
In ice, each water molecule forms hydrogen bonds with 4 other water molecules to yield a regular lattice structure (3.4 in liquid water), and thus ice has lower density than liquid water.
The polarity and hydrogen-bonding capability of water make it an excellent solvent for polar molecules.
- good solvent for charged and polar compounds(hydrophilic)
- poor solvent for nonpolar compounds(hydrophobic)
3 Hydrophobic interaction
The hydrophobic interaction is one of the main factors behind:
- Protein folding
- Protein-protein association
- Formation of lipid micelles
- Binding of steroid hormones to their receptors
Long-chain fatty acid in aqueous solution
- Dispersion
- Clusters
- Micelles
Hydrophobic effect favors ligand binding
- Binding sites in enzymes and receptors are often hydrophobic
- Such sites can bind hydrophobic substrates and ligands such as steroid hormones
- Many drugs are designed to take advantage of the hydrophobic effect
4 Van der Waals interaction
- Weak intermolecular forces between molecules as a result of each inducing polarization in the other
- Occur between any two atoms close to each other
- Van der Waals interactions have two components:
- Attractive force: depends on the polarizability
- Repulsive force: depends on the size of atoms
- Attraction dominates at longer distances (typically 0.4-0.7 nm)
- Repulsion dominates at very short distances
- There is a minimum energy distance (van der Waals radius or van der Waals contact distance)
Biochemistry significance
- Weak individually
- Easily broken, reversible
- Universal
- Occur between any two atoms that are near each other
- Importance
- stabilizes biological macromolecules (stacking in DNA)
- facilitates binding of polarizable ligands
Interactions
Covalent interactions
- A covalent bond is formed by the sharing of a pair of electrons between adjacent atoms
- Covalent bonds are the strongest bonds present in biomolecules (100 kcal/mol)
Noncovalent interactions
- Ionic interactions
- Electrostatic interactions between permanently charged species, or between the ion and a permanent dipole
- Hydrogen bonds
- Weak electrostatic interactions between one electronegative atom and a H atom covalently linked to a second electronegative atom
- Van der Waals interactions
- Weak interactions between all atoms, regardless of polarity
- Attractive and repulsive component
- Hydrophobic interactions
- The association of nonpolar groups or compounds with each other in aqueous systems
Noncovalent interactions are much weaker than covalent bonds. They are reversible and weak individually but strong cumulatively.
The most stable macromolecular conformations are those in which hydrogen bonding is maximized within the molecule and between the molecule and the solvent, and in which hydrophobic moieties cluster in the interior of the molecule away from the aqueous solvent.
5 Ionization of water

- Dissociation of water is a rapid reversible process
- Most water molecules remain un-ionized, thus pure water has very low electrical conductivity (resistance: 18 M$\ohm$•cm)
- The equilibrium is strongly to the left
- Extent of dissociation depends on the temperature
equilibrium constant Keq
$$
K_{eq} = \frac{[\mathrm{H}^+][\mathrm{OH}^-]}{[\mathrm{H}2\mathrm{O}]}
$$
Ion product of water
$$
K_w = K{eq} \cdot [\mathrm{H}_2\mathrm{O}]=[\mathrm{H}^+][\mathrm{OH}^-]=1\times 10^{-14}M^2
$$
In pure water
$$
[\mathrm{H}^+]=[\mathrm{OH}^-]=10^{-7}M
$$
pH
$$
pH = -lg[\mathrm{H}^+]
$$
The pH and pOH must always add to 14. In neutral solution, [H+] = [OH-] and the pH is 7.
pH scale: 1 unit = 10-fold
6 Dissociation of weak acids and bases: principle
Weak acids and bases dissociate partially in water. The extent of dissociation is determined by the acid dissociation constant Ka
$$
K_a = \frac{[\mathrm{H}^+][\mathrm{Ac}^-]}{[\mathrm{AcH}]}
$$
pKa indicates the relative strength of a weak acid or base
$$
pK_a = -lgK_a
$$
strong acid has large Ka and small pKa.
Conjugate acid-base pairs consist of a proton donor and a proton acceptor .
To get the final pH, approximation works for sufficiently weak acids and bases
$$
pH = -lg\sqrt{cK_a}
$$
At the midpoint of the titration, the concentrations of the proton donor and proton acceptor are equal, and the pH is numerically equal to the pKa.
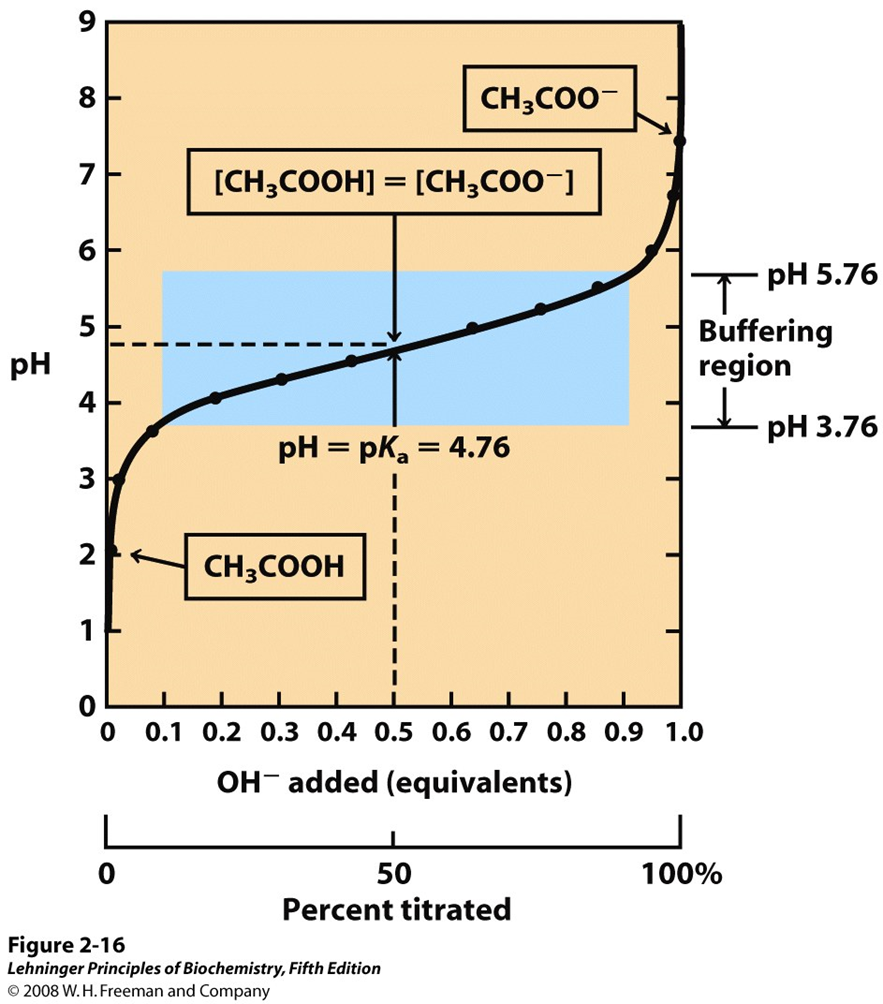
Buffering region = pKa$\pm$1
Henderson-Hasselbalch Equation
$$
p\mathrm{H}= pK_a + lg\frac{[\mathrm{A}^-]}{[\mathrm{HA}]}
$$
7 Buffers are mixtures of weak acids and their conjugate bases
- Buffering capacity of acid/base system is maximal at pH = pKa
- Buffering capacity is lost when the pH differs from pKa by more than 1 pH unit
Biological buffer systems
- Buffer systems in vivo are mainly based on
- Phosphate (H2PO4- / HPO42-), acts in the cytoplasm
- Bicarbonate (H2CO3 /HCO3-), important for blood plasma (pH 7.35-7.45)
- histidine, efficient buffer at neutral pH
- Buffer systems in vitro are often based on sulfonic acids of cyclic amines
- HEPES
- PIPES
- CHES
The pKa of the protonated nitrogen of the side chain of histidine is 6.0

summary
- Water is the medium for life.
- Weak, noncovalent interactions are crucial for the structure and function of DNA, RNA and proteins.
- pH = -log[H+]. It provides a standard way to measure the H+ concentration in an aqueous solution.
- pKa is an integral property of an ionizable group. It is the extent of ionization of an ionizable group that varies with the pH of the solution.
- The Henderson-Hasselbalch Equation relates the pH of a solution of a weak acid and its salt to the relative concentrations of the acid and the salt.
- Buffer is a solution of a weak acid and its conjugate base that resists pH changes in biological systems.
Chapter 3 Amino Acids, Peptides, and Proteins
1 Proteins are the main agents of biological function
2 Amino acids: building blocks of proteins
3 Formation of peptides
4 Proteins are polypeptides
5 Working with proteins
Chapter 5 Protein Function
1 Functions of globular proteins
2 Interaction with other molecules
3 Function of myoglobin
4 Function of hemoglobin
5 Two types of the immune systems
Cellular immune system
- targets own cells that have been infected
- also destroys virus particles and infecting bacteria
- key players
- macrophages
- killer T cells(T_c)
- inflammatory T cells(TH_1)
Many infected cells display fragments of infectious particles on their surface.
- Phagocytes
- specialized cells that eat invaders
- Macrophages
- large phagocytes that ingest bacteria
Humoral “fluid” immune system
- targets extracellular pathogens
- can also recognize foreign proteins
- makes soluble antibodies
- keep “memory” of past infections
- key players
- B-lymphocytes
- helper T-cells(TH_2)
Vertebrates fight infections with soluble antibodies that specifically bind antigens.
Antigens are substances that stimulate production of antibodies.
- Typically macromolecular in nature
- Recognized as foreign by immune system
- Coat proteins of bacteria and viruses
- Surface carbohydrates of cells or viruses
Antibodies are proteins that are produced by B cells and specifically bind to antigens
- Binding will mark the antigen for destruction or interfere with its function
- A given antibody will bind to a small region(epitope) of the antigen
- One antigen can have several epitopes
Chapter 6 Enzymes
1 Introduction to enzymes
Enzymes are catalytically active biological macromolecules. They are catalysts of biological systems and catalyze almost every biochemical reaction.